Ensuring data integrity

Paper based records
As industry standards continue to be raised, some approaches that were considered acceptable in the past will no longer support you in achieving compliance. Paper based records can be particularly problematic, as they do not provide the same safeguards as electronic batch records.ALCOA+
With Ci-DMS, data integrity has been designed in from the outset. From automated data capture and timestamping, to tamperproof recordkeeping, it ensures that all your manufacturing data meets the principles of ALCOA+ (ALCOA+ is the extended set of data integrity principles described in regulatory guidance).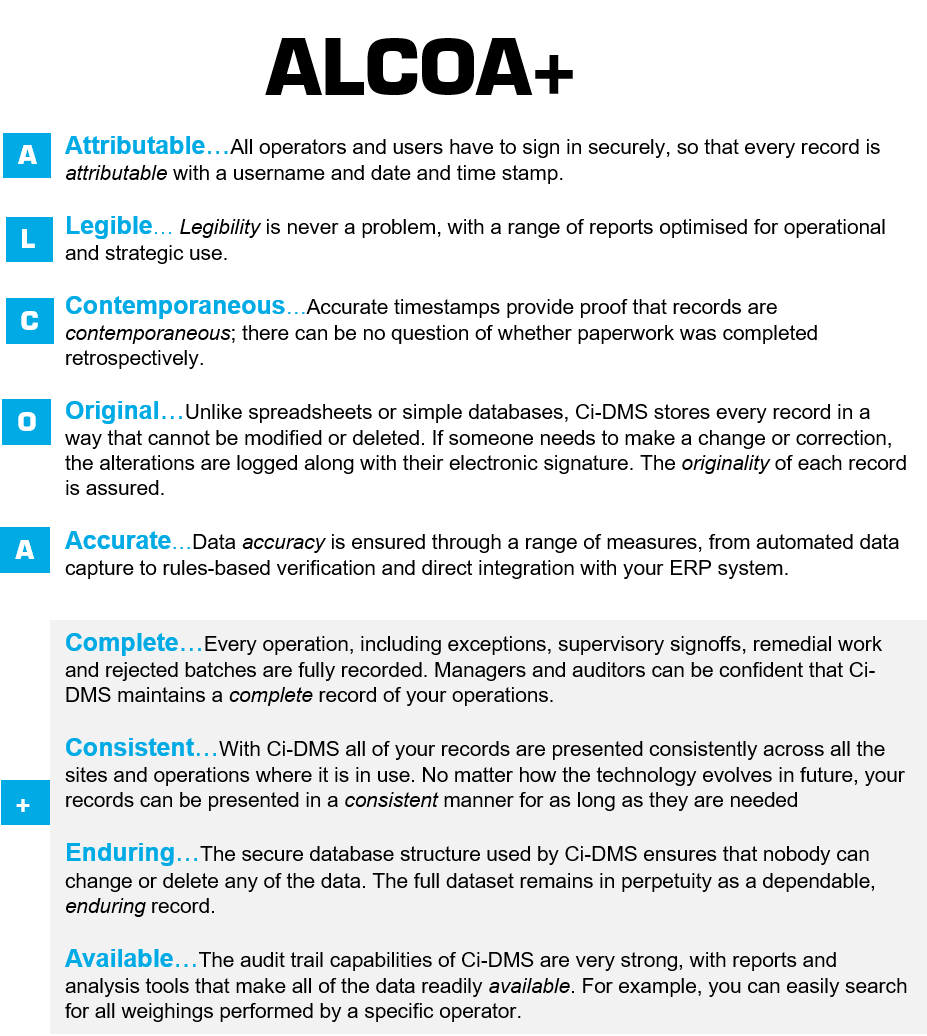
Help Topics
Explore More
Continue Your Research
Individual OSD weight sorting is a vital process for quality control
Pharmaceutical R&D, clinical trials and manufacture all require strict standards of uniformity, and weight is..
Contact UsExplore our reliable MES solutions
Our powerful, customisable and efficient Ci-DMS MES helps you tackle even the most exacting and demanding production requirements to ensure high production quality and excellent regulatory compliance.
More5 Factors to Consider When Choosing Your MES Partner
Selecting the right partner is as important as choosing the right MES solution. We understand..
Contact UsHow you can meet us
Request a meeting, or find us at an exhibition
Request a meetingUpcoming exhibitions