Eliminating human error

Catching errors early
When an operator strays from standard operating procedure or makes a mistake in recording, the impact can be minimised if it is identified and rectified right away. However, if an error does go unnoticed the cost implications can be substantial. Even a small mistake can result in an entire batch being rejected, with all the material, production resources and staff time wasted.The limitations of paper
With paper-based instructions and records, there is no easy way of checking whether operators have started to take shortcuts that expose you to mistakes, nor whether they are completing records diligently, or treating them as a check-box exercise. With the volume of information and detail in paper batch records, it is very hard for anyone to spot anomalies or variations from standard operating procedure. It is only during the final batch approval process that errors are likely to be picked-up.
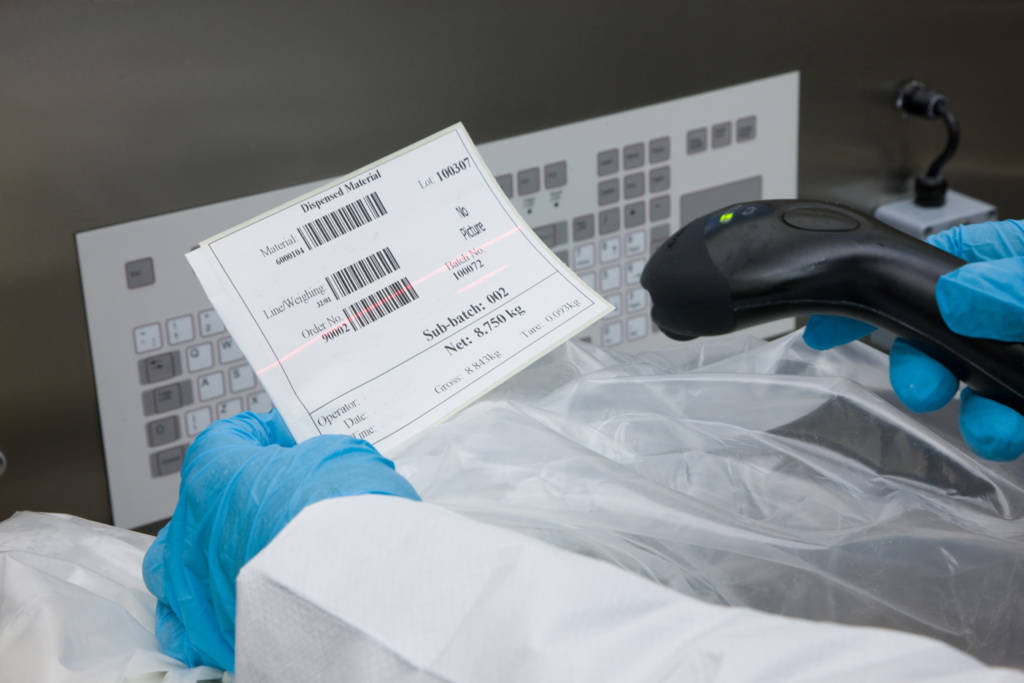
Automating operations and recordkeeping
With an MES system, such as Ci-DMS, the most error-prone manual operations are automated, together with checking and recordkeeping. The chances of errors are minimised as manual operations are automated and all data entry is validated, whether it is from a barcode, an integrated piece of equipment or the keyboard. If Ci-DMS registers any discrepancies, they are flagged-up immediately and the process halted, preventing the risk of production delay and batch failure.Supporting checking and supervision
Any MES will keep detailed, accurate batch records; with Ci-DMS this information is presented to auditors and QPs in a form that makes it much easier to review, so they can work quickly while having the greatest chance of spotting any anomalies. Ci-DMS helps you identify any problematic procedures that need to be redesigned, as well as highlighting any operators who need additional training or intervention.
Help Topics
Explore More
Continue Your Research
Individual OSD weight sorting is a vital process for quality control
Pharmaceutical R&D, clinical trials and manufacture all require strict standards of uniformity, and weight is..
Contact UsExplore our reliable MES solutions
Our powerful, customisable and efficient Ci-DMS MES helps you tackle even the most exacting and demanding production requirements to ensure high production quality and excellent regulatory compliance.
More5 Factors to Consider When Choosing Your MES Partner
Selecting the right partner is as important as choosing the right MES solution. We understand..
Contact UsHow you can meet us
Request a meeting, or find us at an exhibition
Request a meetingUpcoming exhibitions