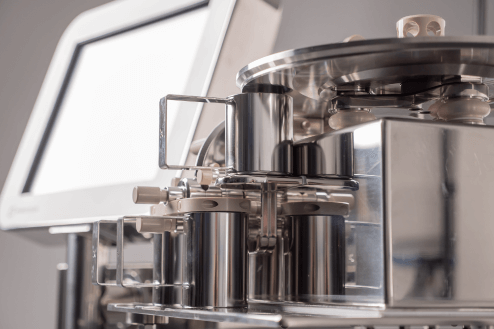
Ci-DMS MES in the Dispensing Suite
Automate your dispensary operation and integrate stock and production order information with your ERP

The cost of maintaining and storing records
Paper-based pharmaceutical manufacturers spend more money than their electronic-based competitors on the cost of maintaining and storing records, as required by the FDA, (estimated $400 Billion Cost of Goods Sold (COGS*).
With Ci-DMS MES batch manufacturing records are maintained and stored electronically with version control and approval. Our customers benefit from electronic traceability enabling quicker audit preparation, and rapid response to audit questions.
*source: various
Achieve compliance whilst improving efficiency
Ci-DMS MES electronically manages the weighing and dispensing of manufacturing and packaging materials and the sampling of raw material lots.
It is used in healthcare environments worldwide to improve Good Manufacturing Practice (GMP).
It replaces paper-based processes, helping achieve better compliance while improving operational efficiency and productivity.
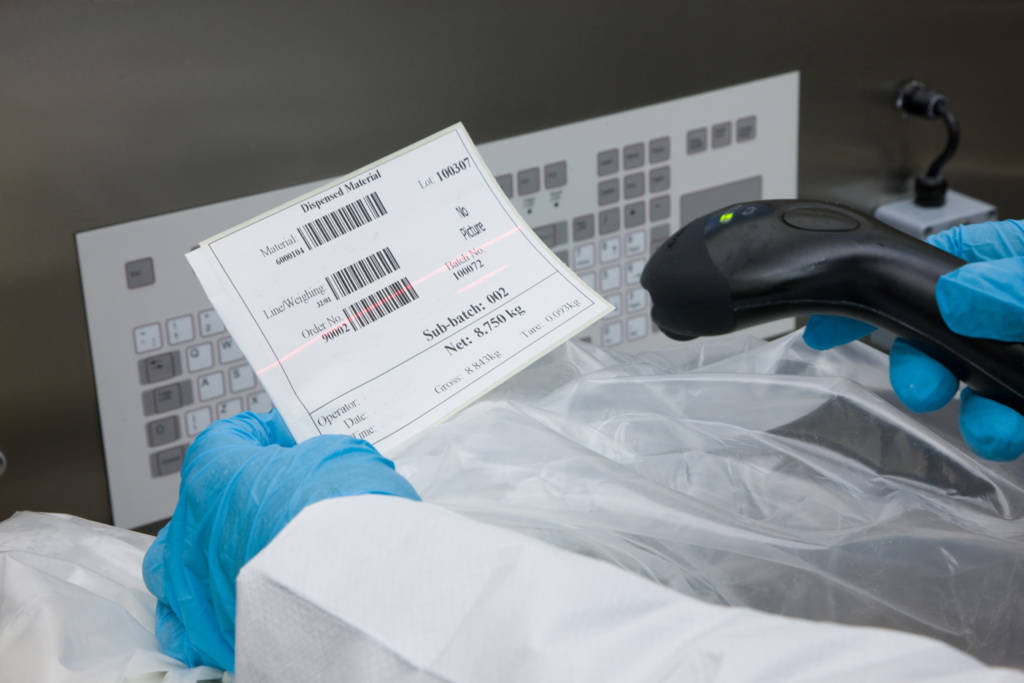
Ci-DMS MES in action
Explore More
Continue Your Research
Solve modern industry challenges with CI Precision
From our industry-leading precision weight sorters to advanced MES solutions, our products and services have been engineered to help you address and overcome your unique industry challenges.
MoreDownload the Ci-DMS MES brochure
Learn more about our Ci-DMS MES — our powerful, versatile, and modular software for paperless manufacturing that helps you tackle even the most exacting and demanding production requirements.
DownloadAutomated, high-precision weight sorting for superior quality control
When Harwell Dosimeters wanted to control the manufacturing quality of their Alanine Pellets they chose CI Precision’s weight sorters to accurately weigh each pellet.
Contact UsHow you can meet us
Request a meeting, or find us at an exhibition
Request a meetingUpcoming exhibitions