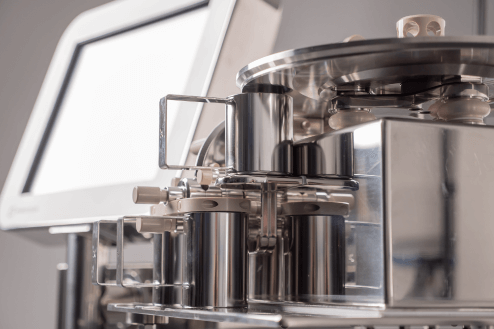
Increase Productivity with our Expert Validation Services
Always run at peak performance with support from the industry’s trusted equipment and services provider
Industry-leading Validation Service Packages
We have supplied weight sorters, check weighers, and MES systems to the pharmaceutical and healthcare industries for over 30 years, so our products and services have been finely tailored for these sectors. We specialise in supporting pharmaceutical businesses with their verification processes from development and testing to materials traceability and supplier selection.
Because of our extensive experience in the sector, we understand that installing and setting up equipment correctly is critical to optimising performance and demonstrating compliance. That’s why we offer a full range of validation service packages to ensure you get the level of support you need.
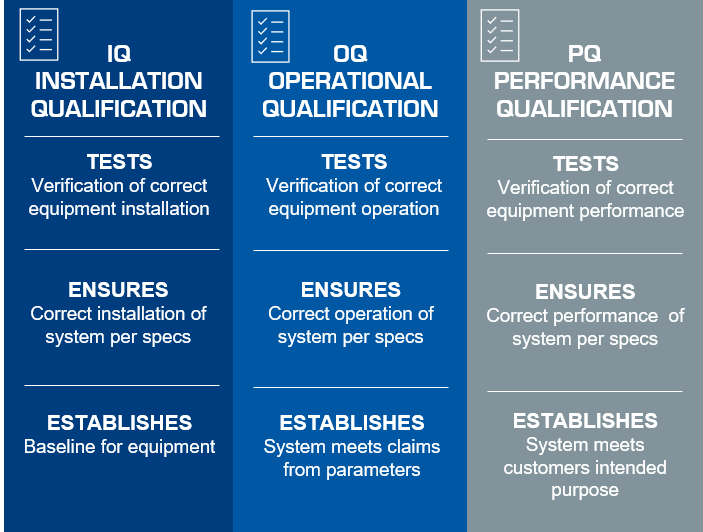


Installation & IQ Support
Every new weight sorter includes installation, IQ support service and operator training by qualified engineers from CI Precision or our partners. We also offer additional OQ/PQ support services to ensure performance and operational compliance.OQ Support
We offer on-site OQ support to help clients validate the functionality of their weight sorters. Our engineers carry out extensive performance checks during normal running and alarm conditions as well as on the data management module to ensure that every critical function of the new weight sorter is operating as intended and meeting functional requirements.PQ Support
CI Precision’s PQ support service is designed to suit your specific needs. We can supply a standard PQ template, which can then be tailored to your requirements. Alternatively, we can assist you with the development of a bespoke PQ document. We can also provide on-site support to assist you as required.Start-up Right the First Time with CI Precision
With over thirty years’ experience supplying weight sorters to the pharmaceutical industry, our specialist team can provide the level of service you need to get your weight sorter up and running quickly, efficiently and cost-effectively.
Enjoy Expert Support Anytime, Anywhere
Get the right support wherever and whenever you need it. Whether you need assistance with regulatory-compliant documentation or require an engineer on-site, our experts are always available to support your quality control, validation, and regulatory compliance requirements.

Maximise Your Return on Investment
Minimise your time spent on OQ/PQ test protocols with our knowledgeable customer service team. With extensive experience and knowledge of the equipment and software, our team can help you get the installation and setup right the first time.Benefit from Working with the Original Equipment Manufacturer
Enjoy outstanding hardware and software support directly from us — the original designers and manufacturers. We know our equipment and software better than anyone else, so we have the working knowledge to ensure that your equipment is always compliant and meeting industry standards as well as operating and performing according to specification.

Drive Productivity with Proven Solutions
Work with our subject matter experts and use our verified, expert-developed OQ/PQ templates to reduce the time and money spent learning, designing and executing test protocols. With our industry-leading expertise, our specialist team can also help you design and implement your own tailored documentation.Explore More
Continue Your Research
Explore our comprehensive support services
From project enquiry through to configuration and after-sales, we offer industry-leading support every step of the way with all our products to ensure a successful project.
MoreAutomated, high-precision weight sorting for superior quality control
When Harwell Dosimeters wanted to control the manufacturing quality of their Alanine Pellets they chose CI Precision’s weight sorters to accurately weigh each pellet.
Contact UsMaximising batch recoveries from R&D scale through to full production levels
As a large pharmaceutical manufacturer, Synthon Chile required equipment that would help them meet the requirements of certain markets and Internal Positive Controls.
Contact UsNext Steps
To find out more contact CI Precision or your local channel partner
Get contact detailsMeet us at an exhibition