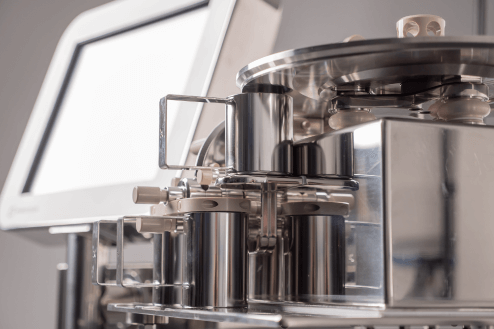
MES for pharmaceutical weigh and dispense
Ci-DMS is a proven software solution for controlling and automating all your pharmaceutical weighing and dispensing operations. It ensures operators follow every process step, checking every detail and maintaining fully compliant records.
With Ci-DMS, your operators are guided faultlessly through every step of weighing and dispensing, with on-screen prompts, connected weighing machines, barcode scanning and automatic label printing. It ensures your recordkeeping is fully ALCOA compliant, and it shares data with your other MES and ERP systems.
Ci-DMS is a configurable software solution which is designed to be delivered, integrated and validated quickly, to provide rapid payback and strong ROI.
From large scale manufacturers to research organisations and producers of personalised medicines, Ci-DMS is providing the assurance and evidence that every ingredient is weighed, dispensed and managed correctly.
Unlike most MES providers, the team behind Ci-DMS is focused on delivering outstanding capabilities in just one area, weigh and dispense. Because this is our entire focus, we offer the optimal solution, addressing all your weighing and dispensing challenges fully and efficiently.
However, Ci-DMS is also designed to be a tightly integrated part of your MES and ERP ecosystem, so you can achieve outstanding ROI without compromise.
Reducing cost by
OVER 50%
Achieving ROI in
<9 MONTHS
Why Implement Ci-DMS in Your Dispensary?
Achieve rapid ROI with a focused solution, delivering an integral part of your MES strategy
![]()
Ensure fully compliant weighing and dispensing
![]()
Maintain complete and accurate records
![]()
Reduce costs of labour and second checking
![]()
Tight integration with ERP and dispensing equipment
![]()
Outstanding project and validation support
![]()
Excellent track record for successful, on-time delivery

Outstanding performance and commitment to excellence
Your team demonstrated exceptional professionalism, ensuring that all milestones were met as per the agreed-upon timeline. This level of commitment and dedication is truly impressive. Furthermore, I would like to extend my gratitude for the extended support provided beyond project completion. Your team was readily available to address any post-implementation concerns or queries we had. I believe it is important to recognize outstanding performance, and I am truly grateful for your team’s commitment to excellence.
Darakhshan Afzal, Reckitt Pakistan

Automation and Process Control
With Ci-DMS, all activities in your dispensary are tightly controlled. Operators are guided through each step in the process, with clear on-screen instructions and checks that ensure adherence to GMP.
Integrated weighing equipment and barcode scanners allow the system to verify that every dispensing operation uses the correct ingredients in exactly the right quantity. And labels are printed automatically so every container can be identified and tracked onward. Read more >
ALCOA Compliant Recordkeeping
Ci-DMS takes away the need for time-consuming, error-prone manual recordkeeping and second checking.
Every step of every operation is tracked and recorded automatically, along with data from each weighing and the batch details of every ingredient used. This ALCOA+ compliant data is kept in a secure database where it can be readily accessed for reporting and auditing. Read more >


Seamless Integration to MES and ERP
Ci-DMS is designed to be an integral part of your MES and ERP ecosystem.
With its dedicated integration module, you can share live data easily and securely with your other systems and equipment. And as your IT landscape evolves, Ci-DMS can be easily configured to share updates with new ERP and MES systems in whatever format they need, with minimal work and validation. Read more >
Explore in more depth
Read more about Ci-DMS and its capabilities in our product paper, MES for pharmaceutical weigh and dispense.
Delivering Project Success
We are proud of our outstanding track record, delivering successful MES projects with rapid ROI

Project Experience
We have been delivering pharmaceutical MES projects for over 40 years with outstanding customer satisfaction and retention. Our project managers and developers are highly capable and experienced in this demanding environment.

Pharma Focus
Ci-DMS has been optimised for pharmaceutical weigh and dispense, without compromise.

Validation Expertise
Ci-DMS projects go live without delay, thanks to our highly regarded validation support and documentation.

Outstanding Customer Support
From training staff to planning disaster recovery measures, our support services cover every eventuality to keep your dispensary running smoothly. All our support people have excellent product and sector knowledge and are fast to respond when you need them.

We have found in CI a great supplier
It took us about 6.5 years to get at this point in which we have found in CI a great supplier. Or in a fact more reliable ‘partner’ in business. Thanks to all those at CI who helped us in this interesting period in our company’s development. With a few data import actions still to do, we have meanwhile started dispensing with DMS for our productions. A big milestone. Now it is time to start exploring the advantages of the system and to improve our processes.
Application Portfolio Manager, Tiofarma

Contact our expert team
Reach out today to discover how our focused MES software and 40+ years of expertise in pharmaceutical weighing and dispensing solutions can help you ensure compliance and maximise ROI.